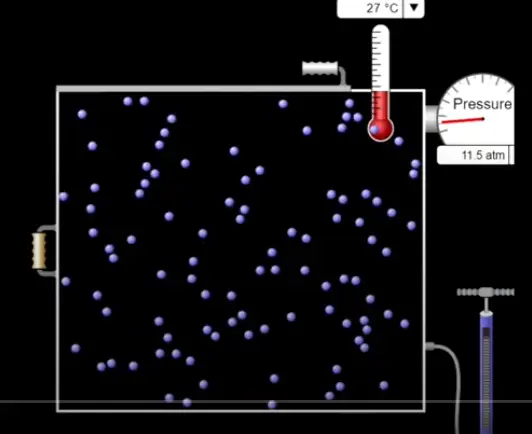
The Maxwell-Boltzmann DistributionWhat is the Maxwell-Boltzmann Distribution? The Maxwell-Boltzmann Distribution Points to notice What is the Maxwell-Boltzmann Distribution?All the molecules of a particular chemical, compound or element have the same mass, so their kinetic energy is only dependent on the speed of the particles. Remember Kinetic Energy = ½mv2 In any particular mixture of moving molecules, the speed will vary a great deal, from very slow particles (low energy) to very fast particles (high energy). Most of the particles however will be moving at a speed very close to the average. The Maxwell-Boltzmann distribution shows how the speeds (and hence the energies) of a mixture of moving particles varies at a particular temperature. The Maxwell-Boltzmann DistributionPoints to notice:
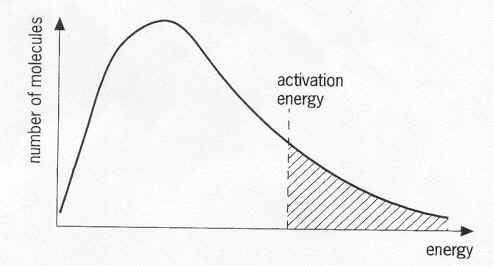
For the reaction to occur, the particles involved need a minimum amount of energy - the Activation energy (EACT). If a particle is not in the shaded area, then it will not have the required energy so it will not be able to participate in the reaction.
| ||
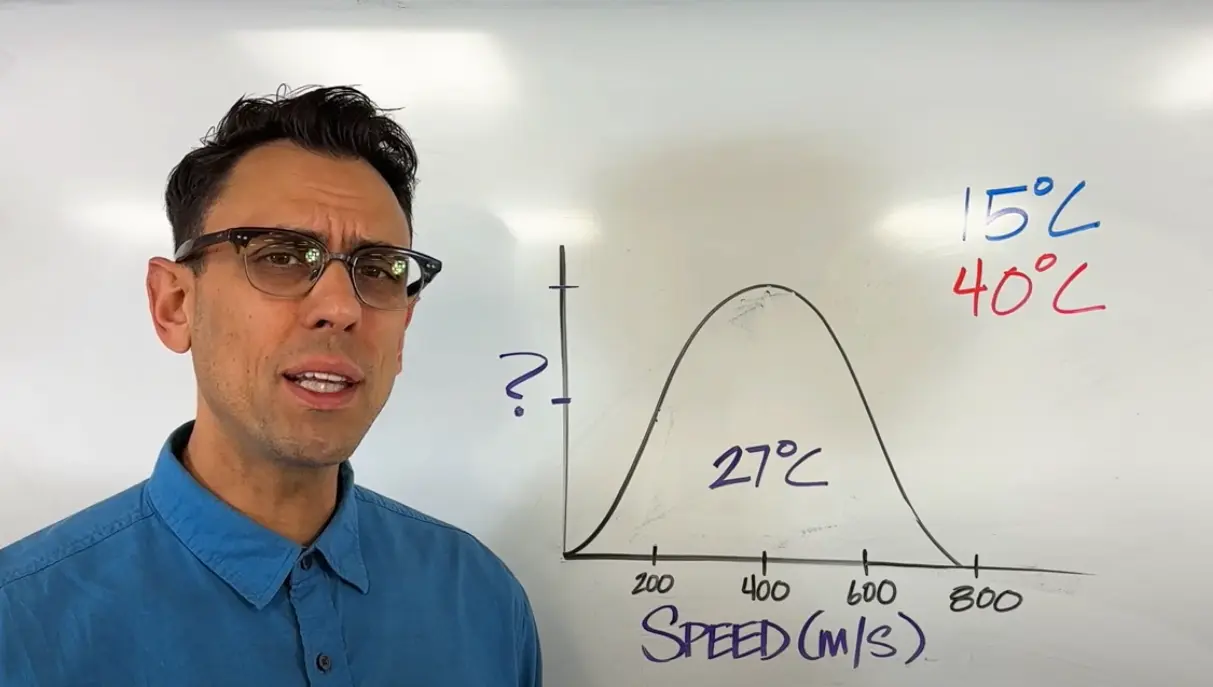
Maxwell Boltzmann Distributions - What the graphs look like and what they mean.
A look at the graphs for these with an explanation of why they look the way that they do. Also a look at how different factors can affect it.
Also have a couple of spots open for tutoring.
Creating places that enhance the human experience.